LONSURF alone in mGC or GEJ cancer
LONSURF alone was studied in the TAGS trial.1,2
TAGS was a multinational, randomized, double-blind, placebo-controlled, Phase 3 trial (N=507).2
Inclusion criteria2
- Age ≥18 years (≥20 years in Japan)
- Histologically confirmed, nonresectable metastatic gastroesophageal junction (GEJ) or gastric cancer
- ECOG performance status (PS) of 0 or 1
- Received postoperative adjuvant chemotherapy and radiotherapy, or pre- and postoperative adjuvant chemotherapy if they had recurrence <6 months of completion of the adjuvant chemotherapy
-
Received at least 2 prior regimens, including:
- Fluoropyrimidine
- Platinum agent
- Taxane and/or irinotecan
- HER2 therapy in patients with HER2-positive tumors
- Were refractory to/intolerant of last prior therapy
2:1 Randomization2
LONSURF + best supportive care (n=337)
35 mg/m2/dose (up to 80 mg/dose) twice daily after meals on Days 1-5 and 8-12 of each 28-day cycle
Placebo + best supportive care (n=170)
Twice daily after meals on Days 1-5 and 8-12 of each 28-day cycle
After the first dose of study medication, all patients were followed for progression at scheduled 8-week intervals with computed tomography (CT) until progression. After the end of treatment, all patients were followed up for survival every 4 weeks until death or loss to follow-up, or until the targeted number of events (deaths) was met.2
Key baseline characteristics2
| Baseline characteristics of patients in the TAGS trial2 | LONSURF (n=337) | Placebo (n=170) |
|---|---|---|
| Age, years | ||
| <65 | 54% | 56% |
| Sex | ||
| Male | 75% | 69% |
| Female | 25% | 31% |
| ECOG PS | ||
| 0 | 36% | 40% |
| 1 | 64% | 60% |
| HER2 status | ||
| Positive | 20% | 16% |
| Negative | 61% | 62% |
| Number of prior therapies | ||
| 2 prior therapies | 37% | 38% |
| 3 prior therapies | 40% | 35% |
| ≥4 prior therapies | 23% | 27% |
| Primary site | ||
| Gastric | 71% | 71% |
| GEJ | 29% | 28% |
| Number of metastatic sites | ||
| 1-2 | 46% | 42% |
| ≥3 | 54% | 58% |
| Prior systemic treatments | ||
| Platinum therapy | 100% | 100% |
| Fluoropyrimidine-based therapy | >99%* | 100% |
| Taxane therapy† | 92% | 87% |
| Irinotecan therapy† | 54% | 58% |
| Ramucirumab therapy | 34% | 32% |
| Anti-HER2 therapy | 18% | 14% |
| Immunotherapy (anti-PD-1/PD-L1) | 7% | 4% |
| Prior treatments | ||
| Gastrectomy | 44% | 44% |
*One patient did not receive a fluoropyrimidine.
†All patients received irinotecan or taxane or both.
PRIMARY ENDPOINT
Help extend survival in metastatic gastric or GEJ cancer with LONSURF alone
Overall Survival (OS) (N=507)1-3
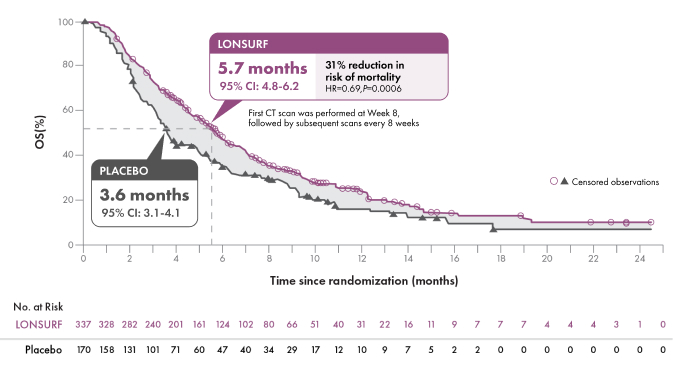
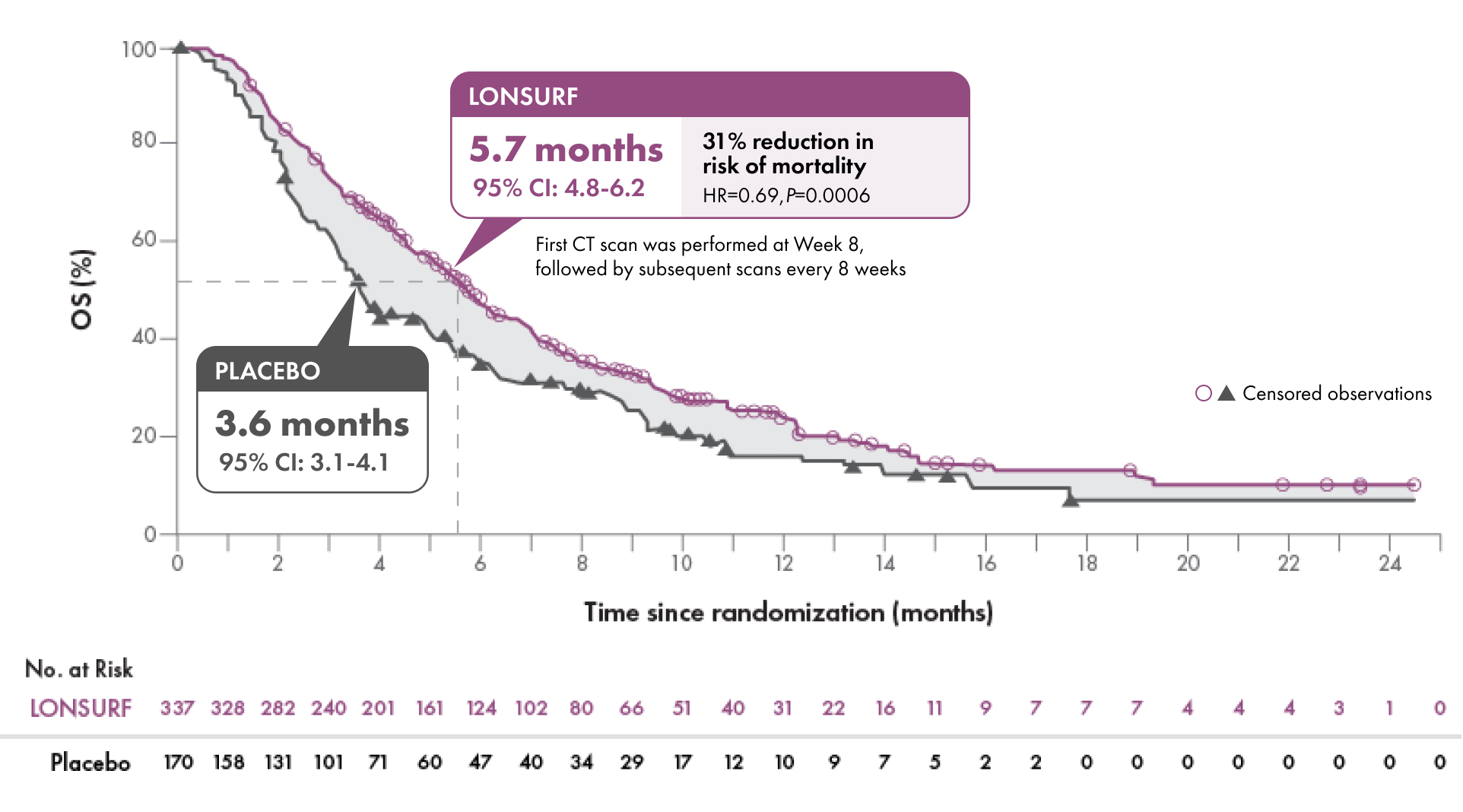
SECONDARY ENDPOINTS
Help prolong progression-free survival
Progression-free Survival1-3
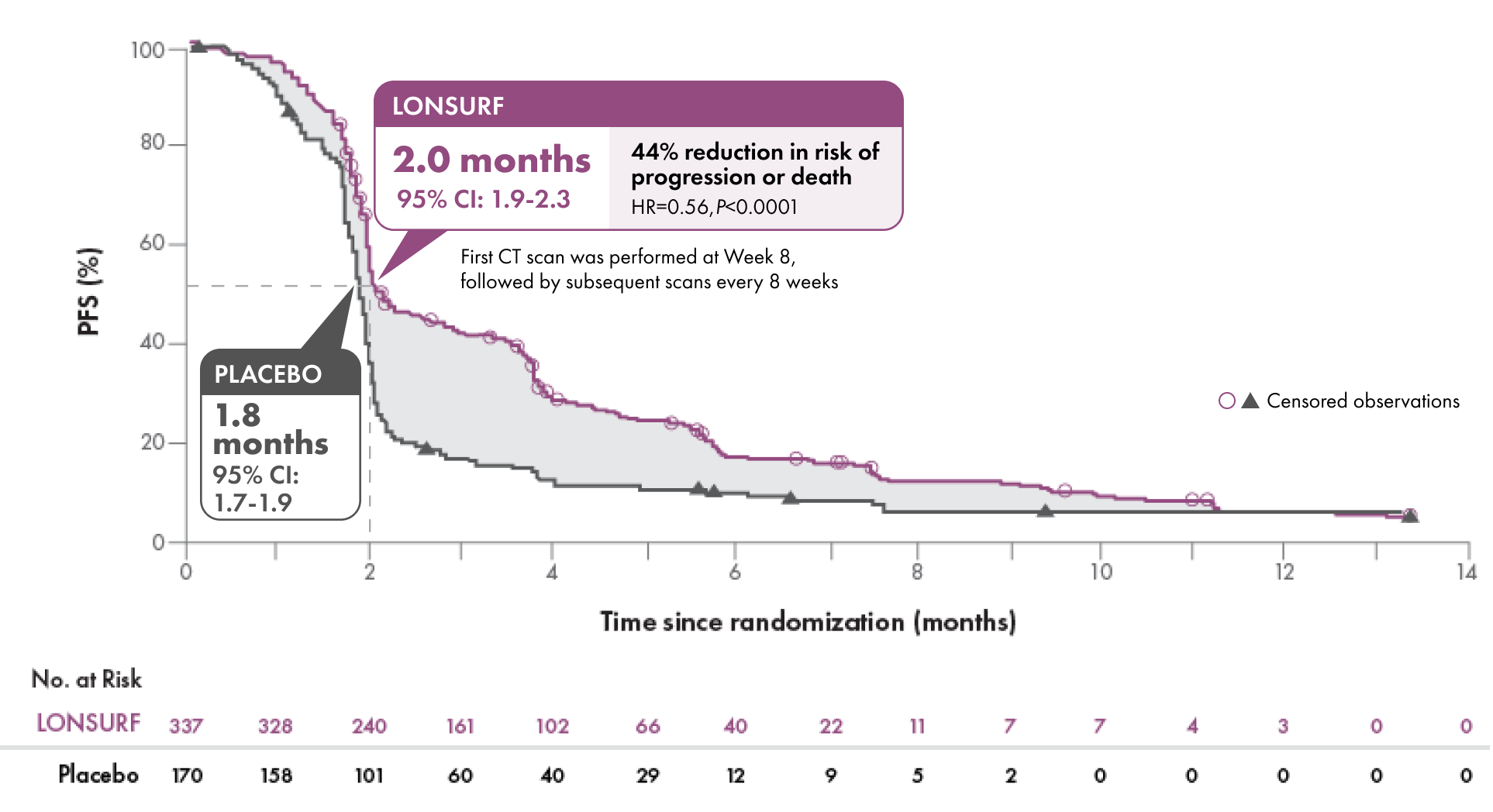
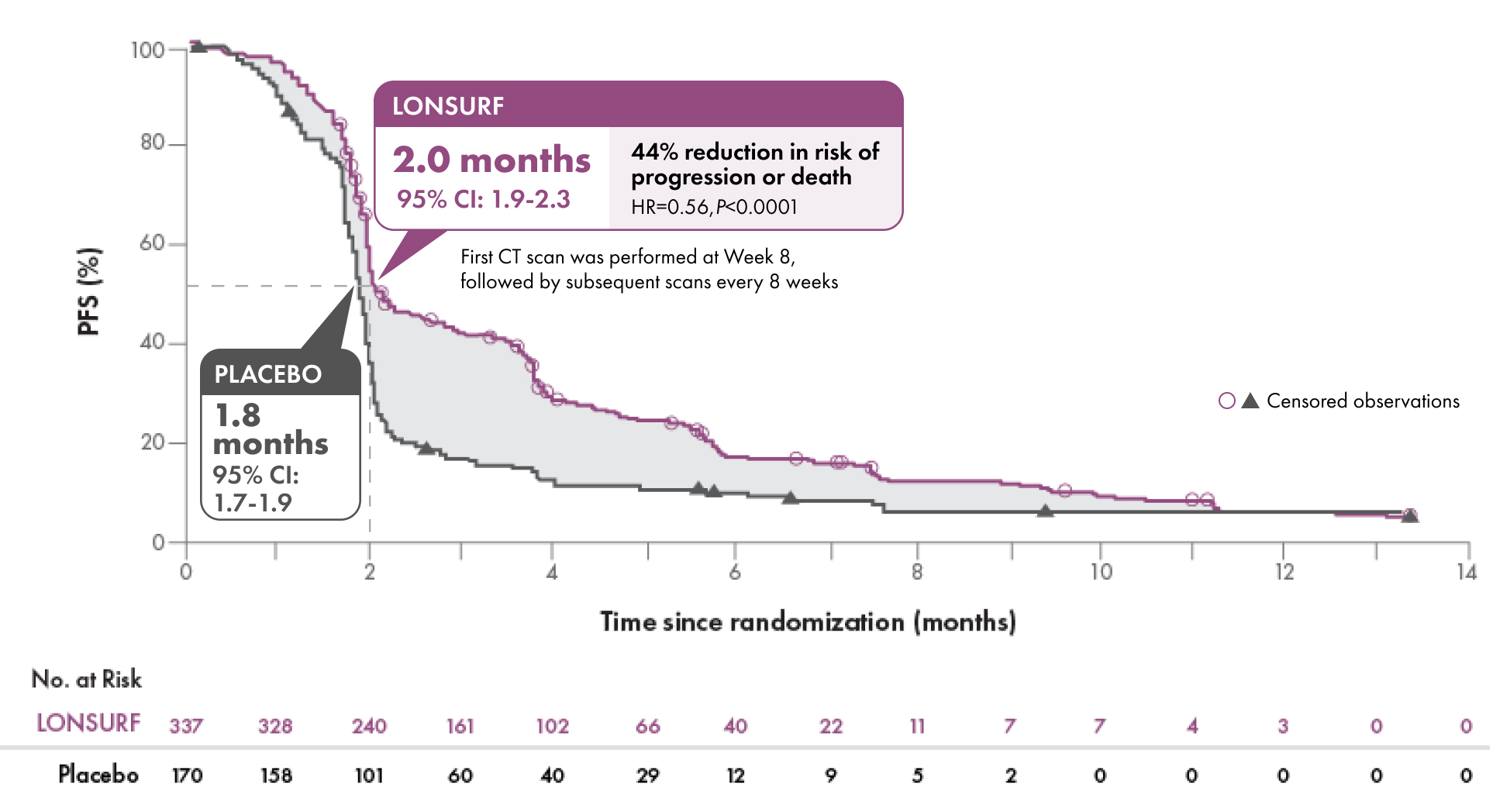
Disease control rate (DCR)2,3
| LONSURF (n=290) | Placebo (n=145) | |
|---|---|---|
| DCR | 44% (128) | 14% (21) |
| Complete response | 0% (1) | 0% (0) |
| Partial response | 4% (12) | 2% (3) |
| Stable response | 40% (115) | 12% (18) |
DCR=complete response + partial response + stable disease.
Time to worsening of ECOG PS
Median Time to Worsening of ECOG PS to ≥22

NCCN Guidelines® in metastatic gastric cancer or GEJ adenocarcinoma
NCCN
Recommended
NCCN Category 1
The National Comprehensive Cancer Network® (NCCN®) recommends trifluridine and tipiracil (LONSURF) as a Category 1 third-line or subsequent treatment option for certain patients with metastatic gastric cancer or GEJ adenocarcinoma.4,5
Category 1=based upon high-level evidence (≥1 randomized Phase 3 trials or high-quality, robust meta-analyses), there is uniform NCCN consensus (≥85% support of the Panel) that the intervention is appropriate.
NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
LONSURF is indicated as a single agent or in combination with bevacizumab for the treatment of adult patients with metastatic colorectal cancer previously treated with fluoropyrimidine‑, oxaliplatin‑ and irinotecan‑based chemotherapy, an anti‑VEGF biological therapy, and if RAS wild‑type, an anti‑EGFR therapy.
LONSURF is indicated for the treatment of adult patients with metastatic gastric or gastroesophageal junction adenocarcinoma previously treated with at least two prior lines of chemotherapy that included a fluoropyrimidine, a platinum, either a taxane or irinotecan, and if appropriate,


LONSURF is indicated as a single agent or in combination with bevacizumab for the treatment of adult patients with metastatic colorectal cancer previously treated with fluoropyrimidine‑, oxaliplatin‑ and irinotecan‑based chemotherapy, an anti‑VEGF biological therapy, and if RAS wild‑type, an anti‑EGFR therapy.
LONSURF is indicated for the treatment of adult patients with metastatic gastric or gastroesophageal junction adenocarcinoma previously treated with at least two prior lines of chemotherapy that included a fluoropyrimidine, a platinum, either a taxane or irinotecan, and if appropriate,
Severe Myelosuppression: In the 1114 patients who received LONSURF as a single agent, LONSURF caused severe or life‑threatening myelosuppression (Grade 3‑4) consisting of neutropenia (38%), anemia (17%), thrombocytopenia (4%) and febrile neutropenia (3%). Three patients (0.3%) died due to neutropenic infection/sepsis; four other patients (0.5%) died due to septic shock. A total of 14% of patients received granulocyte‑colony stimulating factors. In the 246 patients who received LONSURF in combination with bevacizumab, LONSURF caused severe or life-threatening myelosuppression (Grade 3‑4) consisting of neutropenia (52%), anemia (5%), thrombocytopenia (4%) and febrile neutropenia (0.4%). One patient (0.4%) died due to abdominal sepsis and two other patients (0.8%) died due to septic shock. A total of 29% of patients received granulocyte-colony stimulating factors. Obtain complete blood counts prior to and on Day 15 of each cycle of LONSURF and more frequently as clinically indicated. Withhold LONSURF for severe myelosuppression and resume at the next lower dosage.
Embryo‑Fetal Toxicity: LONSURF can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to the fetus. Advise females of reproductive potential to use effective contraception during treatment and for at least 6 months after the final dose.
USE IN SPECIFIC POPULATIONSLactation: It is not known whether LONSURF or its metabolites are present in human milk. There are no data to assess the effects of LONSURF or its metabolites on the breastfed child or the effects on milk production. Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment with LONSURF and for 1 day following the final dose.
Male Contraception: Because of the potential for genotoxicity, advise males with female partners of reproductive potential to use condoms during treatment with LONSURF and for at least 3 months after the final dose.
Geriatric Use: Patients 65 years of age or older who received LONSURF as a single agent had a higher incidence of the following hematologic laboratory abnormalities compared to patients younger than 65 years: Grade 3 or 4 neutropenia (46% vs 32%), Grade 3 anemia (20% vs 14%), and Grade 3 or 4 thrombocytopenia (6% vs 3%). Patients 65 years of age or older who received LONSURF in combination with bevacizumab had a higher incidence of the following hematologic laboratory abnormalities compared to patients younger than 65 years: Grade 3 or 4 neutropenia (60% vs 46%) and Grade 3 or 4 thrombocytopenia (5% vs 4%).
Renal Impairment: No adjustment to the starting dosage of LONSURF is recommended in patients with mild or moderate renal impairment (CLcr of 30 to 89 mL/min). Reduce the starting dose of LONSURF for patients with severe renal impairment (CLcr of 15 to 29 mL/min) to a recommended dosage of 20 mg/m2.
Hepatic Impairment: Do not initiate LONSURF in patients with baseline moderate or severe (total bilirubin > 1.5 times ULN and any AST) hepatic impairment. Patients with severe hepatic impairment (total bilirubin > 3 times ULN and any AST) were not studied. No adjustment to the starting dosage of LONSURF is recommended for patients with mild hepatic impairment.
ADVERSE REACTIONSSerious adverse reactions occurred in 25% of patients. The most frequent serious adverse reactions (≥2%) were intestinal obstruction (2.8%), and COVID-19 (2%). Fatal adverse reactions occurred in 1.2% of patients who received LONSURF in combination with bevacizumab, including rectal fistula (0.4%), bowel perforation (0.4%) and atrial fibrillation (0.4%).
The most common adverse reactions or laboratory abnormalities (≥10% in incidence) in patients treated with single‑agent LONSURF at a rate that exceeds the rate in patients receiving placebo in mCRC: anemia (77% vs 33%), neutropenia (67% vs 0.8%), asthenia/fatigue (52% vs 35%), nausea (48% vs 24%), thrombocytopenia (42% vs 8%), decreased appetite (39% vs 29%), diarrhea (32% vs 12%), vomiting (28% vs 14%), abdominal pain (21% vs 19%), and pyrexia (19% vs 14%). In metastatic gastric cancer or gastroesophageal junction (GEJ): neutropenia (66% vs 4%), anemia (63% vs 38%), nausea (37% vs 32%), thrombocytopenia (34% vs 9%), decreased appetite (34% vs 31%), vomiting (25% vs 20%), infections (23% vs 16%) and diarrhea (23% vs 14%).
Pulmonary emboli occurred more frequently in LONSURF‑treated patients compared to placebo: in mCRC (2% vs 0%) and in metastatic gastric cancer and GEJ (3% vs 2%).
Interstitial lung disease (0.2%), including fatalities, has been reported in clinical studies and clinical practice settings in Asia.
The most common adverse reactions or laboratory abnormalities (≥20% in incidence) in patients treated with LONSURF in combination with bevacizumab vs LONSURF alone were neutropenia (80% vs 68%), anemia (68% vs 73%), thrombocytopenia (54% vs 29%), fatigue (45% vs 37%), nausea (37% vs 27%), increased aspartate aminotransferase (34% vs 28%), increased alanine aminotransferase (33% vs 23%), increased alkaline phosphate (31% vs 36%), decreased sodium (25% vs 20%), diarrhea (21% vs 19%), abdominal pain (20% vs 18%), and decreased appetite (20% vs 15%).
References: 1. LONSURF [package insert]. Princeton, NJ: Taiho Oncology, Inc.; 2023. 2. Shitara K, Doi T, Dvorkin M, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19(11):1437-1448. 3. Data on file. Taiho Oncology, Inc., Princeton, NJ. 4. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Gastric Cancer V.2.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed June 5, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org. 5. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Esophageal and Esophagogastric Junction Cancers V.3.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed June 5, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org.
