How LONSURF +/- bevacizumab works
LONSURF is an antimetabolite combination agent1,2
- Trifluridine (FTD) is a fluorinated thymidine analogue that is incorporated into the DNA and causes DNA dysfunction that leads to the death of cancer cells1,3*
- FTD is not a prodrug of 5-FU4
- Tipiracil (TPI) inhibits the rapid degradation of trifluridine1
Bevacizumab is a vascular endothelial growth factor (VEGF) inhibitor5
- VEGF inhibitors prevent angiogenesis (the growth of new blood vessels) by selectively binding to circulating VEGF and thus inhibiting the binding of VEGF to its cell surface receptors (VEGFR)6
Proposed mechanism of action1,3,5,7
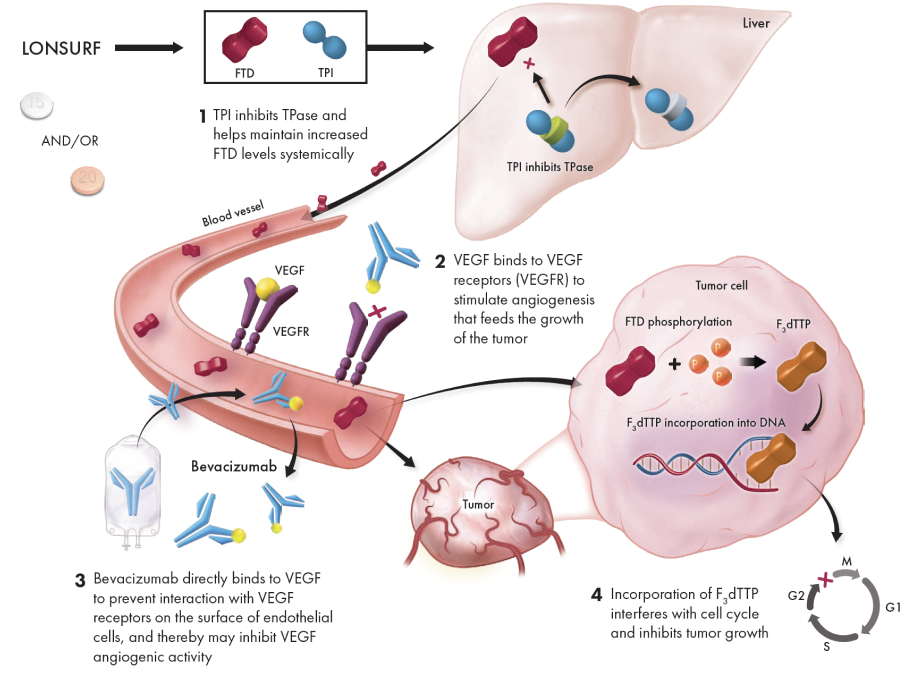
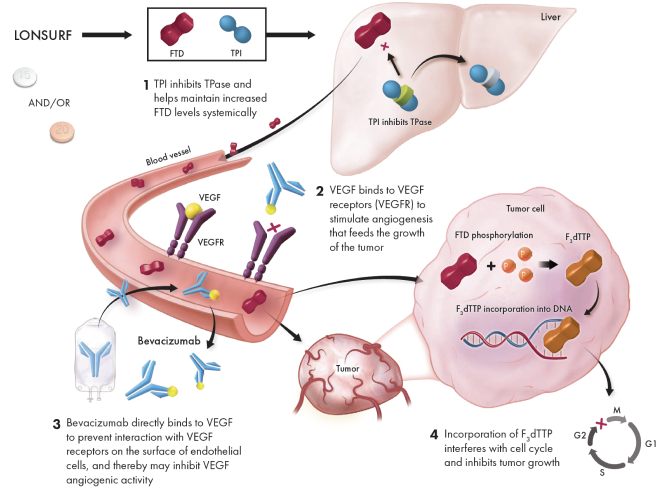
LONSURF interferes with DNA synthesis and prevents cell proliferation, while bevacizumab starves the tumor by preventing angiogenesis1,5
This presentation is for illustrative purposes only.
Not intended to imply clinical significance.
*LONSURF can also have toxic effects on healthy, non-tumor cells.
5-FU=5-fluorouracil; F3dTTP=FTD-triphosphate.
References: 1. LONSURF [package insert]. Princeton, NJ: Taiho Oncology, Inc.; 2023. 2. Uboha N, Hochster HS. TAS-102: a novel antimetabolite for the 21st century. Future Oncol. 2016;12(2):153-163. 3. Matsuoka K, limori M, Niimi S, et al. Trifluridine induces p53-dependent sustained G2, phase arrest with its massive misincorporation into DNA and few DNA strand breaks. Mol Cancer Ther. 2015;14(4):1004-1013. 4. Lenz H-J, Stintzing S, Loupakis F. TAS-102, a novel antitumor agent: a review of the mechanism of action. Cancer Treat Rev. 2015;41(9):777-783. 5. Avastin [package insert]. South San Francisco, CA: Genentech, Inc.; 2022. 6. Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25(4):581-611. 7. Mayer RJ, Van Cutsem E, Falcone A, et al; RECOURSE Study Group. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372(20):1909-1919.
INDICATIONS
LONSURF is indicated as a single agent or in combination with bevacizumab for the treatment of adult patients with metastatic colorectal cancer previously treated with fluoropyrimidine‑, oxaliplatin‑ and irinotecan‑based chemotherapy, an anti‑VEGF biological therapy, and if RAS wild‑type, an anti‑EGFR therapy.
LONSURF is indicated for the treatment of adult patients with metastatic gastric or gastroesophageal junction adenocarcinoma previously treated with at least two prior lines of chemotherapy that included a fluoropyrimidine, a platinum, either a taxane or irinotecan, and if appropriate,


INDICATIONS
LONSURF is indicated as a single agent or in combination with bevacizumab for the treatment of adult patients with metastatic colorectal cancer previously treated with fluoropyrimidine‑, oxaliplatin‑ and irinotecan‑based chemotherapy, an anti‑VEGF biological therapy, and if RAS wild‑type, an anti‑EGFR therapy.
LONSURF is indicated for the treatment of adult patients with metastatic gastric or gastroesophageal junction adenocarcinoma previously treated with at least two prior lines of chemotherapy that included a fluoropyrimidine, a platinum, either a taxane or irinotecan, and if appropriate,
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Severe Myelosuppression: In the 1114 patients who received LONSURF as a single agent, LONSURF caused severe or life‑threatening myelosuppression (Grade 3‑4) consisting of neutropenia (38%), anemia (17%), thrombocytopenia (4%) and febrile neutropenia (3%). Three patients (0.3%) died due to neutropenic infection/sepsis; four other patients (0.5%) died due to septic shock. A total of 14% of patients received granulocyte‑colony stimulating factors. In the 246 patients who received LONSURF in combination with bevacizumab, LONSURF caused severe or life-threatening myelosuppression (Grade 3‑4) consisting of neutropenia (52%), anemia (5%), thrombocytopenia (4%) and febrile neutropenia (0.4%). One patient (0.4%) died due to abdominal sepsis and two other patients (0.8%) died due to septic shock. A total of 29% of patients received granulocyte-colony stimulating factors. Obtain complete blood counts prior to and on Day 15 of each cycle of LONSURF and more frequently as clinically indicated. Withhold LONSURF for severe myelosuppression and resume at the next lower dosage.
Embryo‑Fetal Toxicity: LONSURF can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to the fetus. Advise females of reproductive potential to use effective contraception during treatment and for at least 6 months after the final dose.
USE IN SPECIFIC POPULATIONS
Lactation: It is not known whether LONSURF or its metabolites are present in human milk. There are no data to assess the effects of LONSURF or its metabolites on the breastfed child or the effects on milk production. Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment with LONSURF and for 1 day following the final dose.
Male Contraception: Because of the potential for genotoxicity, advise males with female partners of reproductive potential to use condoms during treatment with LONSURF and for at least 3 months after the final dose.
Geriatric Use: Patients 65 years of age or older who received LONSURF as a single agent had a higher incidence of the following hematologic laboratory abnormalities compared to patients younger than 65 years: Grade 3 or 4 neutropenia (46% vs 32%), Grade 3 anemia (20% vs 14%), and Grade 3 or 4 thrombocytopenia (6% vs 3%). Patients 65 years of age or older who received LONSURF in combination with bevacizumab had a higher incidence of the following hematologic laboratory abnormalities compared to patients younger than 65 years: Grade 3 or 4 neutropenia (60% vs 46%) and Grade 3 or 4 thrombocytopenia (5% vs 4%).
Renal Impairment: No adjustment to the starting dosage of LONSURF is recommended in patients with mild or moderate renal impairment (CLcr of 30 to 89 mL/min). Reduce the starting dose of LONSURF for patients with severe renal impairment (CLcr of 15 to 29 mL/min) to a recommended dosage of 20 mg/m2.
Hepatic Impairment: Do not initiate LONSURF in patients with baseline moderate or severe (total bilirubin > 1.5 times ULN and any AST) hepatic impairment. Patients with severe hepatic impairment (total bilirubin > 3 times ULN and any AST) were not studied. No adjustment to the starting dosage of LONSURF is recommended for patients with mild hepatic impairment.
ADVERSE REACTIONS
Serious adverse reactions occurred in 25% of patients. The most frequent serious adverse reactions (≥2%) were intestinal obstruction (2.8%), and COVID-19 (2%). Fatal adverse reactions occurred in 1.2% of patients who received LONSURF in combination with bevacizumab, including rectal fistula (0.4%), bowel perforation (0.4%) and atrial fibrillation (0.4%).
The most common adverse reactions or laboratory abnormalities (≥10% in incidence) in patients treated with single‑agent LONSURF at a rate that exceeds the rate in patients receiving placebo in mCRC: anemia (77% vs 33%), neutropenia (67% vs 0.8%), asthenia/fatigue (52% vs 35%), nausea (48% vs 24%), thrombocytopenia (42% vs 8%), decreased appetite (39% vs 29%), diarrhea (32% vs 12%), vomiting (28% vs 14%), abdominal pain (21% vs 19%), and pyrexia (19% vs 14%). In metastatic gastric cancer or gastroesophageal junction (GEJ): neutropenia (66% vs 4%), anemia (63% vs 38%), nausea (37% vs 32%), thrombocytopenia (34% vs 9%), decreased appetite (34% vs 31%), vomiting (25% vs 20%), infections (23% vs 16%) and diarrhea (23% vs 14%).
Pulmonary emboli occurred more frequently in LONSURF‑treated patients compared to placebo: in mCRC (2% vs 0%) and in metastatic gastric cancer and GEJ (3% vs 2%).
Interstitial lung disease (0.2%), including fatalities, has been reported in clinical studies and clinical practice settings in Asia.
The most common adverse reactions or laboratory abnormalities (≥20% in incidence) in patients treated with LONSURF in combination with bevacizumab vs LONSURF alone were neutropenia (80% vs 68%), anemia (68% vs 73%), thrombocytopenia (54% vs 29%), fatigue (45% vs 37%), nausea (37% vs 27%), increased aspartate aminotransferase (34% vs 28%), increased alanine aminotransferase (33% vs 23%), increased alkaline phosphate (31% vs 36%), decreased sodium (25% vs 20%), diarrhea (21% vs 19%), abdominal pain (20% vs 18%), and decreased appetite (20% vs 15%).

